About the Bridget Nesko Ovarian Cancer Project
Imagine if just one percent of the nearly 200,000 women who will be diagnosed with ovarian cancer over the next decade in the U.S. could be warned about the disease’s presence in their bodies by as little as one year before it develops into its next stage.
Or, if as little as one percent of the more than 132,700 women who will die in the next decade from ovarian cancer in the U.S. could be spared that end result, or have their lives extended because they were able to react to an early warning about their illness.
Imagine if you had the chance to provide early diagnosis or intervention to your mother, sister, wife, partner, daughter or yourself, and you were unable to do so because of lack of awareness.
Would it matter?
While there are several simple tests and procedures used to detect testicular cancer in males at early stages of development, there are no “authorized” tests made clinically available to women for early warnings about ovarian cancer, unless they have a family history of the disease.
One procedure – a simple blood test called a “Cancer Antigen 125″ (or “CA-125”) can help to measure the possible ovarian cancer presence at a very early stage of its development. It is used, in fact, as a leading early warning test in a number of nations outside of the U.S.
While the U.S. medical community in general has mixed reactions about the use of the CA-125 test in early ovarian cancer detection, they have no such concerns about its use, once ovarian cancer has been diagnosed, or as a means of evaluating a patient’s progress as they proceed through chemotherapy treatment.
As the widower of a late-diagnosed ovarian cancer victim (despite clear and early evidence of multiple symptoms before her actual clinical diagnosis at stage three-c), I firmly believe that women have the right to arm themselves with the possible knowledge they can gain with early testing. Kevin Nesko
Had Bridget Nesko – a nurse by profession – had access to a team of aware medical professionals who could “connect the dots” by understanding the signs and symptoms of her emerging ovarian cancer, she could have insisted on having a CA-125 blood test. That test would have indicated a cancer’s presence, leading her to further examination, testing and earlier diagnosis and treatment.
About the Bridget Nesko Ovarian Cancer Project
According to the Mayo Clinic and the Memorial Sloan Kettering Cancer Center, ovarian cancer rarely has noticeable symptoms when it is in its earliest stages. As the cancer progresses, subtle symptoms begin to appear, but still may not be noticed or may be blamed on other common conditions, such as constipation or an irritable bowel.
The symptoms of ovarian cancer include:
- Abdominal bloating or swelling.
- Pain in the abdomen, back, kidney area or pelvis.
- Difficulty eating or feeling full quickly.
- Lack of appetite.
- Needing to – or having the feeling of needing to – urinate frequently.
- Change in bowel habits (constipation or diarrhea).
- Change in menstrual periods.
- Vaginal bleeding between periods.
- Unintentional weight gain or loss.
Although the symptoms of ovarian cancer may be vague, particularly in the early stages, they are usually fairly constant and represent a change from how a woman may normally feel. Symptoms also worsen as the cancer progresses.
BEING PROACTIVE IN DEFYING OVARIAN CANCER
The Bridget Nesko Ovarian Cancer Project is presently evaluating the ways and means of implementing a revolutionary pilot program called “Project KOCA”. Under “Project KOCA”, women would be provided with a free and simple “Ovarian Cancer Symptoms Journal“, and asked to track apparent symptoms they may be having that could indicate the presence of ovarian cancer. If they have three or more of the key symptoms over a greater than four-week period, they are advised to take the journal to their family physician or gynecologist and request a simple CA-125 blood test.
Under Project KOCA, free CA-125 testing access would be made available by the Bridget Nesko Ovarian Cancer Project to candidates unable to afford such tests, and/or who are unable to locate a family physician, nurse practitioner, gynecologist, or oncologist willing to do such testing. We can also provide the names of medical professionals familiar with ovarian cancer symptoms who are willing to proscribe such tests, if appropriate.
About the “Cancer Antigen 125” blood test
A CA 125 test measures the amount of the protein CA 125 (cancer antigen 125) in the blood. Traditionally, the test may be used to monitor certain cancers during and after treatment. In some situations, the test may be used to look for early signs of ovarian cancer in people with a very high risk of the disease, have a family history of the disease, or have several of the tell-tale symptoms that suggest presence of the disease.
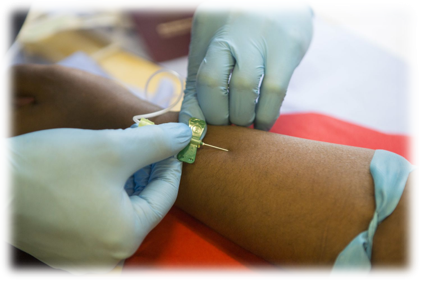
A convoluted argument by the healthcare industry: While many in the medical community suggest that a CA 125 test isn’t accurate enough to use for ovarian cancer screening in general because many conditions can increase the level of this protein, the test is used to track progress in chemotherapy treatment of the disease, and to test if ovarian cancer has returned to patients after treatment appeared to have “paused” progression of the disease.
Our question is, if a CA-125 test us useful in assessing the progress of treatment, or to check for cancer recurrence, why would it not be effective as a an “early warning” tool if ovarian cancer is suspected?
Some in the medical community say because some conditions that can cause an increase in CA 125 include many that aren’t cancerous, such as menstruation and uterine fibroids. Certain cancers may also cause an increased level of CA 125, including endometrial, peritoneal and fallopian tube cancers.
Diagnosis of these conditions, however, would also be beneficial to a woman seeking answers and possible early diagnosis.
What happens if your CA-125 test result comes back with a “relatively high” reading?
As part of our pilot program, a copy of your CA 125 test will be provided to YOU and your health care provider, who will discuss the results with you. If your CA 125 level is higher than expected, you may have a condition that isn’t cancerous, or the test result could mean that you have ovarian, endometrial, peritoneal or fallopian tube cancer. Your medical provider may recommend other tests and procedures to determine your diagnosis.
Generally speaking, the normal range of CA-125 is considered to be 0-35 units/mL, while a level above 35 units/mL is considered to be a high CA-125 level.
A number of conditions that aren’t cancerous can cause an elevated CA 125 level, including: Endometriosis, Liver disease, Menstruation, Pelvic inflammatory disease, Pregnancy and Uterine fibroids If you’ve been diagnosed with ovarian, endometrial, peritoneal or fallopian tube cancer, a decreasing CA 125 level often indicates that the cancer is responding to treatment. A rising level may indicate a return or continued growth of the cancer.
Other tests that your medical team will use to determine the likelihood of your having ovarian cancer would include non-invasive procedures, including: a medical history debrief and physical exam by a medical practitioner; consultation with a specialist; imaging tests, including an ultrasound, computed tomography (CT) scans, a barium enema x-ray, a magnetic resonance imaging (MRI) scan, a Chest x-ray (to evaluate possible spread if suspected), and finally, a positron emission tomography (PET) scan.
What Are Your Next Steps?
First, see our page The Truth About Ovarian Cancer.
If you have a family history of ovarian cancer, and you have two or more symptoms for more than two weeks, contact your medical professional and share your concern about ovarian cancer.
If you feel you are not being heard, contact us and we can help you to locate a more responsive medical professional. Also, under the pilot program, CA-125 testing would be made available to candidates unable to afford such tests, and who are unable to locate a medical professional willing to offer such testing.
If You Are a Medical Professional
In much of Europe, the administration of a non-invasive CA-125 blood test is considered the “go to” step, once ovarian cancer is suspected. In the U.S., there is resistance to utilizing this step, which we believe contributes unnecessarily to late-stage diagnosis and premature death of ovarian cancer victims.
In order to thoroughly understand the U.S. insurance industry’s and medical community’s perception of using CA-125 blood testing as a non-invasive precautionary step in assessing the possible presence of ovarian cancer in a patient with a history of the disease in their family or who is experiencing a number of the signs and symptoms of early ovarian cancer’s presence, we are hosting both a series of confidential Focus Groups and establishing a permanent “Feedback Panel” of medical professionals.
For more information and to apply to be a member of a Focus Group or Feedback Panel, please Click Here.
If you are a medical professional and would like to join our team as a volunteer member of our Medical Advisory Board, or if you have questions, please feel free to contact us at: Info@BridgetAngelFund.Org, or by phone, at (856) 595 2184.